Description
Product Overview
Retatrutide 10mg is a high-purity, lyophilized peptide developed exclusively for advanced laboratory research. As a triple agonist targeting glucagon-like peptide-1 (GLP-1), glucose-dependent insulinotropic polypeptide (GIP), and glucagon (GCG) receptors, Retatrutide (LY3437943), developed by Eli Lilly and Company, represents a cutting-edge investigational compound for studying metabolic disorders, including obesity, type 2 diabetes, and non-alcoholic fatty liver disease (NAFLD). Supplied as a lyophilized powder in a 3 mL sterile glass vial, this product is formulated with >99.65% purity (verified by HPLC) and is intended solely for in vitro or in vivo non-human research by qualified professionals. Retatrutide 20mg is not approved for human or veterinary use, nor is it intended as a drug, dietary supplement, or cosmetic.
Chemical and Physical Properties
- Chemical Name: Retatrutide (LY3437943, sodium salt form)
- Molecular Formula: C221H342N46O68
- Molecular Weight: 4731.33 g/mol
- CAS Number: 2381089-83-2
- PubChem CID: 485663353
- Amino Acid Sequence: Tyr-{Aib}-Gln-Gly-Thr-Phe-Thr-Ser-Asp-Tyr-Ser-Ile-{α-Me-Leu}-Leu-Asp-Lys-{diacid-C20-gamma-Glu-(AEEA)-Lys}-Ala-Gln-{Aib}-Ala-Phe-Ile-Glu-Tyr-Leu-Leu-Glu-Gly-Gly-Pro-Ser-Ser-Gly-Ala-Pro-Pro-Pro-Ser-NH2
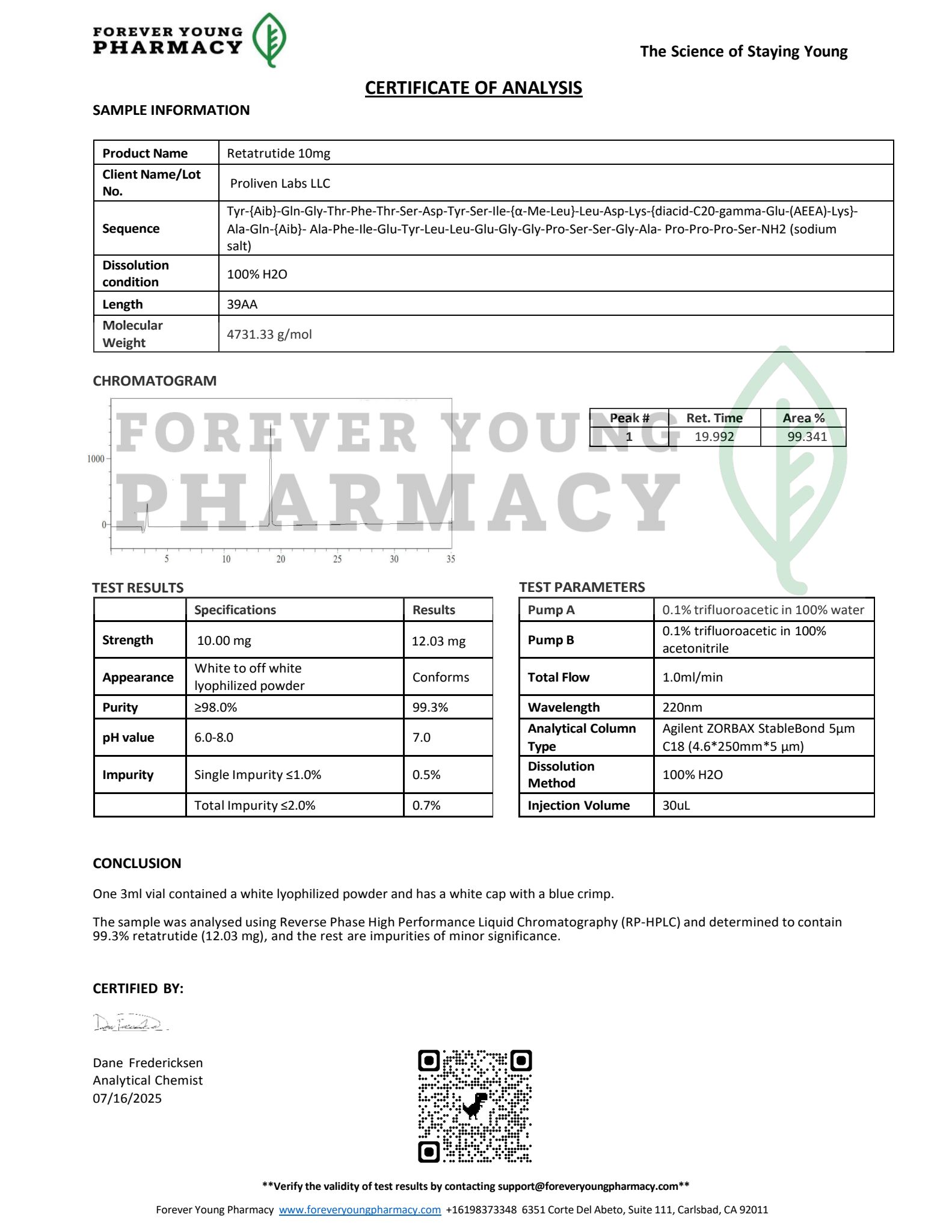
- Purity: >99% (confirmed by High-Performance Liquid Chromatography [HPLC] and Mass Spectrometry [MS])
- Appearance: White to off-white lyophilized powder
- Solubility: Soluble in sterile water, saline, or appropriate aqueous buffers for reconstitution
- Vial Specifications: 10 mg Retatrutide per 5 mL sterile, borosilicate glass vial with rubber stopper and aluminum crimp seal
- pH Stability: Stable in reconstituted solutions with pH 6.5–8.0 (optimal for research applications)
- Half-Life: Approximately 6 days in biological systems, supporting once-weekly dosing in research models
- EC50 Values (Receptor Affinity):
- GLP-1 receptor: 0.0643 nM
- GIP receptor: 0.775 nM
- Glucagon receptor: 5.79 nM
Retatrutide’s unique structure, incorporating a C20 fatty diacid for prolonged activity and non-natural amino acids like α-aminoisobutyric acid (Aib), enhances its stability and receptor-binding efficiency, making it an exceptional tool for metabolic research.
Mechanism of Action
Retatrutide is a GGG (GLP-1/GIP/Glucagon) tri-agonist, simultaneously activating three key metabolic receptors:
- GLP-1 Receptor: Stimulates glucose-dependent insulin secretion, suppresses glucagon release, slows gastric emptying, and reduces appetite, contributing to glycemic control and weight reduction.
- GIP Receptor: Enhances insulin release in response to meals and promotes fat metabolism, amplifying weight loss and improving lipid profiles.
- Glucagon Receptor: Increases energy expenditure by stimulating lipolysis and glycogenolysis, elevating basal metabolic rate and facilitating fat loss.
This synergistic triple-agonist mechanism distinguishes Retatrutide from single-agonist (e.g., semaglutide) or dual-agonist (e.g., tirzepatide) peptides, offering a broader spectrum of metabolic effects. Its long half-life and high receptor potency make it ideal for studying sustained metabolic regulation in research models.
Research Applications
Retatrutide 10mg is designed for laboratory investigations into:
- Obesity and Weight Loss: Phase 2 clinical trials demonstrated dose-dependent weight reduction, with participants achieving:
- 17.5% mean body weight loss (approximately 41 pounds) at 24 weeks with a 12 mg dose.
- 24.2% mean body weight loss (approximately 58 pounds) at 48 weeks with a 12 mg dose, with 83% of participants losing ≥15% of body weight and 26% achieving ≥30% weight loss.
- Type 2 Diabetes Mellitus: Improves insulin sensitivity, reduces HbA1c levels, and enhances glucose homeostasis, making it a valuable tool for diabetes research.
- Non-Alcoholic Fatty Liver Disease (NAFLD): Preliminary data suggest significant reductions in liver fat content (up to 90% of participants achieving normal liver fat levels), offering potential for studying NAFLD and non-alcoholic steatohepatitis (NASH).
- Cardiometabolic Health: Research indicates improvements in systolic blood pressure (up to 8 mmHg reduction), triglycerides, LDL cholesterol, and fasting insulin levels.
- Energy Metabolism: Glucagon receptor activation promotes thermogenesis and fat oxidation, ideal for studying energy expenditure pathways.
- Pharmacokinetics: Its extended half-life supports once-weekly administration protocols in animal models or in vitro systems.
Key Research Findings
- Weight Loss Efficacy: In phase 2 trials, Retatrutide outperformed comparators like semaglutide and tirzepatide in weight reduction, with a linear dose-response relationship up to 12 mg.
- Safety Profile: Mild to moderate gastrointestinal side effects (e.g., nausea, vomiting, diarrhea, constipation) were most common, primarily during dose escalation. No severe hypoglycemia or treatment-related deaths were reported.
- Cardiometabolic Benefits: Significant reductions in HbA1c (up to 2.02%), triglycerides, and LDL cholesterol, alongside improvements in insulin sensitivity and blood pressure.
- Liver Health: Up to 93% of participants with NAFLD achieved normalized liver fat content at higher doses, suggesting potential applications in hepatic research.
Storage and Handling Instructions
- Unreconstituted Storage: Store at -20°C or below in a non-frost-free freezer to maintain stability for up to 24 months. Protect from light and moisture using a desiccated, light-proof container.
- Reconstitution: Reconstitute with 1–2 mL of sterile water, bacteriostatic water, or a suitable aqueous buffer (e.g., phosphate-buffered saline, pH 7.4). Gently swirl to dissolve; avoid vigorous shaking to prevent degradation.
- Post-Reconstitution Storage: Store reconstituted solutions at 2–8°C (refrigerated) and use within 14–28 days to ensure stability. Avoid freezing reconstituted peptide.
- Handling Precautions: Use sterile techniques during reconstitution and handling. Wear appropriate personal protective equipment (PPE), including gloves and lab coat, to avoid contamination.
- Stability Notes: Exposure to temperatures above 25°C or repeated freeze-thaw cycles may compromise peptide integrity. Consult batch-specific Certificate of Analysis (COA) for precise storage recommendations.
Packaging and Quality Assurance
- Packaging: Supplied in a 3 mL clear, sterile glass vial with a rubber stopper and aluminum crimp seal, ensuring product integrity during transport and storage.
- Quality Control: Each batch undergoes rigorous testing, including:
- HPLC analysis for >99% purity.
- Mass spectrometry for molecular weight confirmation.
- Endotoxin testing to ensure absence of pyrogens.
- Sterility testing to confirm compliance with research-grade standards.
- Documentation: Includes a COA, HPLC chromatogram, and MS report with each vial, detailing batch-specific purity, molecular weight, and quality metrics.
- TFA Removal: Trifluoroacetic acid (TFA) is removed during synthesis to minimize interference in sensitive research applications.
Shipping and Delivery
- Cold-Chain Shipping: Shipped with dry ice or gel packs to maintain temperatures at or below -20°C during transit. Special postage fees may apply for fridge-line products.
- Delivery Conditions: Inspect packaging upon receipt for signs of damage or temperature excursions. Contact the supplier immediately if issues are detected.
- International Shipping: Complies with regulations for shipping research peptides. Verify local import restrictions before ordering.
Safety and Regulatory Information
- Intended Use: For laboratory research only by qualified professionals. Not for human or animal consumption, diagnostic, therapeutic, or cosmetic use.
- Regulatory Status: Retatrutide is an investigational compound in phase 3 clinical trials (as of June 29, 2025) and is not approved by the FDA or any other regulatory body for clinical use.
- Safety Precautions:
- Handle in a controlled laboratory environment with proper ventilation and safety equipment.
- Avoid inhalation, ingestion, or skin contact. In case of accidental exposure, follow MSDS guidelines and seek medical advice.
- Store securely, out of reach of children and unauthorized personnel.
- Legal Compliance: Purchasers are responsible for ensuring compliance with local, national, and international regulations governing the purchase, storage, and use of research peptides.
Potential Side Effects
Based on phase 2 trial data in human subjects (for reference in designing animal or in vitro studies):
- Common: Gastrointestinal effects (nausea, vomiting, diarrhea, constipation), particularly during initial dosing or dose escalation.
- Less Common: Transient increases in heart rate (mean 2–3 bpm), mild injection site reactions.
- Rare: Elevated pancreatic enzyme levels (amylase/lipase) without clinical pancreatitis.
- Contraindications in Research: Avoid use in models with hypersensitivity to peptide components or severe renal/hepatic impairment, as these may alter pharmacokinetics.
Research Considerations
- Dosing Protocols: In phase 2 trials, doses ranged from 1 mg to 12 mg weekly, with escalation schedules (e.g., starting at 2 mg, increasing to 4 mg, 8 mg, or 12 mg over weeks). Researchers should tailor dosing to specific study designs.
- Model Systems: Suitable for rodent models (e.g., diet-induced obese mice), cell cultures (e.g., adipocytes, hepatocytes), or organoid systems studying metabolic pathways.
- Analytical Methods: Use HPLC, ELISA, or receptor-binding assays to quantify Retatrutide activity in experimental systems.
- Combination Studies: Explore synergistic effects with other metabolic agents (e.g., insulin, SGLT2 inhibitors) in controlled settings.
Ethical Note: Researchers must adhere to ethical guidelines for animal or in vitro studies, ensuring humane treatment and compliance with institutional review board (IRB) or equivalent oversight.
Disclaimer
Retatrutide 10mg is strictly for research purposes and not for human or veterinary use. The purchaser assumes full responsibility for safe handling, storage, and compliance with all applicable laws. The supplier is not liable for misuse or unauthorized applications. Always consult the provided Material Safety Data Sheet (MSDS), COA, and relevant scientific literature before initiating experiments. For updates on clinical trial data or research protocols, refer to peer-reviewed publications or clinical trial registries (e.g., ClinicalTrials.gov).







Reviews
There are no reviews yet.