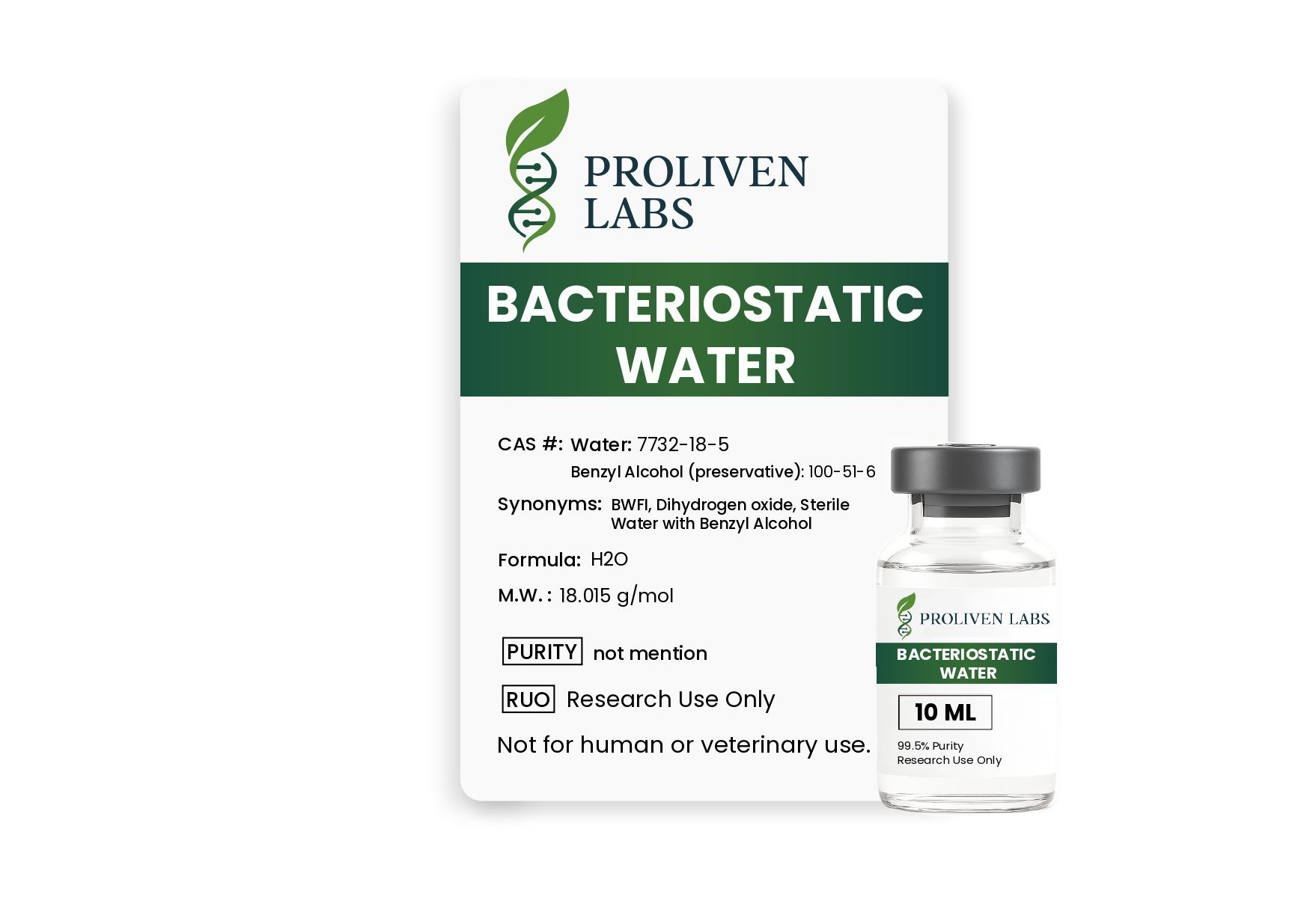
Description
USP Grade | Sterile | Multi-Dose | Preserved with Benzyl Alcohol
Overview
Bacteriostatic Water 10ml is a high-quality, sterile, non-pyrogenic solution designed for the safe and effective reconstitution of medications requiring dilution before injection. Formulated with 0.9% benzyl alcohol as a preservative, it inhibits bacterial growth, allowing for multiple safe withdrawals from the same vial for up to 28 days when stored properly.
Ideal for use in medical, clinical, and research settings, this 10ml vial is a reliable choice for healthcare professionals and patients needing a precise, sterile diluent for injectable medications such as peptides, hormones, or other parenteral drugs.
We also offer premium collagen peptides and vital proteins collagen peptides known for their reliable benefits. Whether you’re looking into peptides for weight loss, muscle growth, or skin health, our high-quality biotech peptides are designed to support a wide range of wellness goals.
Key Features
- Sterile & Non-Pyrogenic: Meets USP (United States Pharmacopeia) standards to ensure high purity and safety.
- Bacteriostatic Agent: Contains 0.9% (9 mg/mL) benzyl alcohol to prevent microbial contamination.
- Multi-Use Vial: Enables multiple withdrawals over 28 days with proper storage.
- pH Range: Approximately 5.7 (range 4.5 to 7.0), ensuring compatibility with a wide range of medications.
- USP-Grade Water: Chemically designated as H₂O and compliant with Water for Injection (WFI) standards.
- Durable Container: The semi-rigid vial is made from a specially formulated polyolefin—a copolymer of ethylene and propylene—tested for biological safety per USP standards. No vapor barrier is required to maintain labeled volume.
- 10ml Volume: Suitable for single or multiple reconstitutions.
- Versatile Use: For diluting or dissolving IV, IM, or SC injectable medications.
Indications and Usage
This parenteral preparation is indicated only for diluting or dissolving drugs intended for intravenous, intramuscular, or subcutaneous injection, as per the manufacturer’s instructions for the medication being administered
Contraindications
- Neonates – Contraindicated in neonates due to the potential toxicity of benzyl alcohol
- Fluid Replacement – Not to be used for fluid replacement therapy
- Epidural/Spinal Use – Not for use in epidural or spinal anesthesia procedures
- Isotonic Requirement – Must be made approximately isotonic before administration
Warnings
- Benzyl alcohol has been associated with toxicity in neonates, including fatal “gasping syndrome”
- Only preservative-free sterile water should be used when preparing medications for neonatal patients
- IV administration without solute may cause hemolysis
- Use aseptic technique when entering and withdrawing from vial
Metabolic Wellness:
- Do not use for IV injection unless additives result in an approximately isotonic admixture
- Follow drug manufacturer instructions for vehicle selection, dilution, route, and rate of administration
- Inspect all reconstituted solutions for clarity, discoloration, or precipitation prior to use
- Pregnancy Category C – Use in pregnancy only if clearly needed; effects on fetus are unknown
- Pediatric Use – Safety and effectiveness not established in pediatric patients; avoid use in neonates
- Drug Compatibility – Consult a pharmacist if unsure about compatibility of additives or benzyl alcohol
- Do not store reconstituted drug solutions unless instructed by drug manufacturer
- Do not use unless the solution is clear and seal is intact
Adverse Reactions
Possible adverse effects may include:
- Febrile response
- Local tenderness
- Abscess formation
- Tissue necrosis or injection site infection
- Venous thrombosis or phlebitis
- Extravasation
If adverse reactions occur, discontinue use, evaluate the patient, take appropriate measures, and if possible, save unused solution for investigation.
Overdosage
This product is unlikely to cause fluid overload, except in very small infants. If fluid overload occurs, reassess patient condition and apply corrective treatment. Refer to Warnings, Precautions, and Adverse Reactions for further guidance
Dosage and Administration
Use volume based on the concentration, dose, and administration route recommended by the drug manufacturer.
Inspect solution visually before use. Do not use if cloudy or if seal is broken. Mix thoroughly and use promptly after reconstitution.
Storage Instructions
Store at room temperature (20–25°C / 68–77°F). Keep out of reach of children. Discard 28 days after first use or if the solution becomes cloudy or shows signs of contamination.
Caution
For use only as a diluent or solvent for medications. Not for direct injection without proper reconstitution. Always consult a healthcare professional before use.




Reviews
There are no reviews yet.