Description
Overview
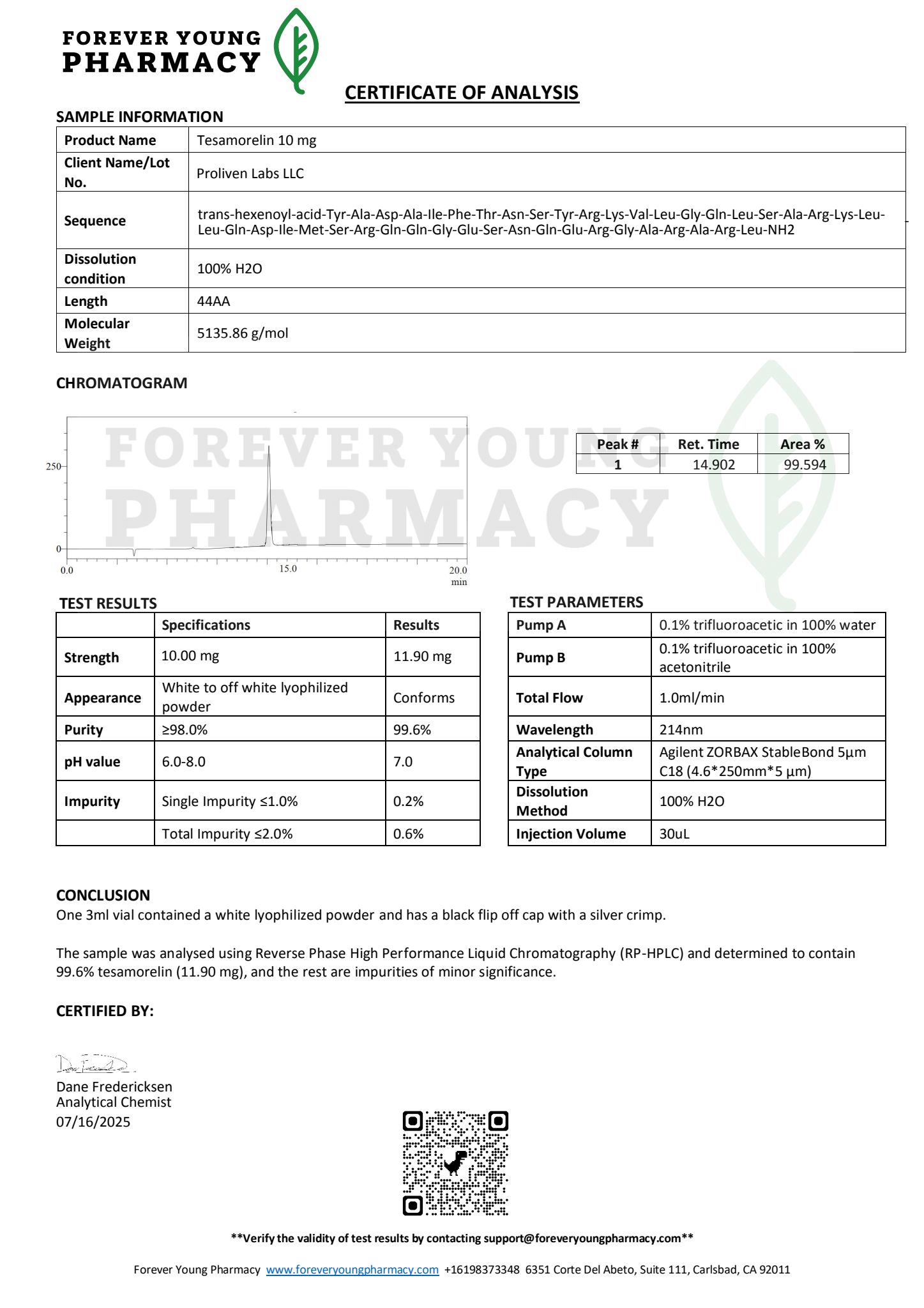
Tesamorelin 10mg is a high-purity, synthetic growth hormone-releasing hormone (GHRH) analog designed to stimulate the pituitary gland to produce and release endogenous growth hormone (GH). This 44-amino-acid peptide, modified with a trans-3-hexanoic acid group for enhanced stability and bioavailability, is FDA-approved for reducing visceral adipose tissue (VAT) in HIV-infected adults with lipodystrophy, a condition characterized by abnormal fat distribution often linked to antiretroviral therapy. Beyond its clinical application, Tesamorelin is widely utilized in research settings to explore its effects on fat metabolism, muscle development, metabolic health, and potential neuroprotective benefits. Supplied as a lyophilized powder for subcutaneous injection, Tesamorelin 10mg is intended for use under medical supervision or for laboratory research by qualified professionals.
Composition and Specifications
- Active Ingredient: Tesamorelin acetate, a synthetic peptide analog of human GHRH with an N-terminal trans-3-hexanoic acid modification to enhance stability.
- Molecular Formula: C221H366N72O67S.
- Molecular Weight: Approximately 5,135.78 g/mol.
- Dosage Form: Lyophilized powder for reconstitution, provided in 10mg vials.
- Purity: ≥99% (pharmaceutical grade for clinical use; research-grade peptides may vary slightly but typically ≥98%).
- Excipients: Mannitol (stabilizer), sucrose, and histidine (in Egrifta formulations).
- Appearance: White to off-white lyophilized powder, free of visible impurities.
- Solubility: Soluble in sterile water or bacteriostatic water for reconstitution.
- pH of Reconstituted Solution: Approximately 5.0–6.0 (neutral to slightly acidic).
- Packaging: Sterile, sealed glass vials containing 10mg of Tesamorelin powder, accompanied by bacteriostatic water (0.9% benzyl alcohol) or sterile water for injection, depending on the formulation (e.g., Egrifta or Egrifta WR). Kits typically include syringes, needles, and alcohol swabs.
- Storage Conditions:
- Unreconstituted Vials: Store at 2°C to 8°C (36°F to 46°F) in the original Medication Box, protected from light and moisture. Egrifta WR vials may also be stored at room temperature (20°C to 25°C or 68°F to 77°F) for up to 3 months.
- Reconstituted Solution: Use immediately after mixing. If not used, refrigerate at 2°C to 8°C and discard within 24 hours. Do not freeze reconstituted solution.
- Research-Grade Storage: For long-term storage, freeze at -20°C to maintain stability for up to 12 months.
- Shelf Life: Typically, 24 months from the date of manufacture when stored properly (check packaging for exact expiration).
Mechanism of Action
Tesamorelin binds specifically to GHRH receptors in the anterior pituitary gland, stimulating the pulsatile release of endogenous growth hormone. This increase in GH triggers the liver to produce insulin-like growth factor-1 (IGF-1), which mediates many of GH’s metabolic effects, including:
- Lipolysis: Breakdown of visceral fat, particularly in the abdominal region.
- Protein Synthesis: Promotion of lean muscle mass and tissue repair.
- Glucose Metabolism: Modulation of insulin sensitivity and energy utilization.
The trans-3-hexanoic acid modification extends Tesamorelin’s half-life (approximately 26–38 minutes in plasma) compared to native GHRH, improving its pharmacokinetic profile. Unlike exogenous GH therapy, Tesamorelin preserves the body’s natural GH pulsatility, reducing the risk of side effects associated with supraphysiological GH levels.
Indications and Uses
- FDA-Approved Indication:
- Reduction of excess visceral abdominal fat in HIV-infected adults with lipodystrophy. Clinical trials (e.g., LIPO-010 and CTR-1011) demonstrated a 15–20% reduction in VAT after 26 weeks of daily 2mg dosing, improving waist circumference, lipid profiles, and cardiovascular risk factors.
- Research Applications:
- Fat Loss: Investigating visceral fat reduction in non-HIV populations, including obesity and metabolic syndrome.
- Metabolic Health: Studying improvements in lipid profiles (e.g., reduced triglycerides, increased HDL), insulin sensitivity, and nonalcoholic fatty liver disease (NAFLD).
- Muscle Development: Exploring lean muscle mass gains, enhanced recovery, and physical performance in athletes or aging populations.
- Neuroprotection: Researching potential benefits in slowing cognitive decline, improving memory, and supporting peripheral nerve regeneration, based on preliminary studies linking GH/IGF-1 to neuroprotection.
- Anti-Aging: Investigating effects on skin elasticity, energy levels, and overall vitality (not FDA-approved).
- Off-Label Use: While not approved for general weight loss or anti-aging, Tesamorelin is sometimes explored off-label for body composition optimization under medical supervision.
Benefits
- Visceral Fat Reduction: Clinically proven to reduce VAT by 15–20% in HIV-associated lipodystrophy, improving body composition and reducing cardiometabolic risk.
- Metabolic Improvements: Enhances HDL cholesterol, reduces triglycerides, and may improve insulin sensitivity in some patients.
- Lean Muscle Support: Promotes protein synthesis, aiding muscle growth and recovery, beneficial for athletes or those with sarcopenia.
- Potential Neuroprotection: Preliminary studies suggest benefits in cognitive function, memory, and peripheral nerve health, particularly in aging populations.
- Preserves GH Pulsatility: Stimulates natural GH release, minimizing risks associated with exogenous GH administration.
- Improved Quality of Life: Patients report better body image, energy levels, and physical function in clinical trials.
Side Effects
- Common (≥5% of patients):
- Injection site reactions (erythema, pruritus, pain, swelling).
- Arthralgia (joint pain), myalgia (muscle pain), or peripheral edema.
- Headache, fatigue, or mild nausea.
- Elevated blood glucose or mild glucose intolerance.
- Less Common (<5%):
- Carpal tunnel syndrome, muscle stiffness, or paresthesia.
- Insomnia or mood changes.
- Serious (Rare):
- Hypersensitivity reactions (rash, urticaria, angioedema, anaphylaxis).
- Increased IGF-1 levels, potentially stimulating tumor growth in patients with a history of malignancy.
- Worsening of glucose intolerance or new-onset diabetes.
- Pituitary-related issues (e.g., headache or visual changes suggestive of pituitary enlargement).
- Management of Side Effects:
- For injection site reactions, apply a cold compress and rotate sites.
- Monitor blood glucose regularly, especially in diabetic or prediabetic patients.
- Discontinue use and seek medical attention for signs of hypersensitivity or severe adverse effects.
Contraindications
- Active malignancy or history of cancer (due to potential IGF-1-mediated tumor growth).
- Hypersensitivity to Tesamorelin or any excipients (e.g., mannitol).
- Pregnancy or breastfeeding (Category X; may cause glucose intolerance or fetal harm).
- Pituitary gland disorders (e.g., tumors, prior surgery, or radiation).
- Acute critical illness (e.g., post-surgery, trauma, or respiratory failure).
Precautions
- Glucose Monitoring: Tesamorelin may increase blood glucose levels. Patients with diabetes or prediabetes should monitor fasting glucose and HbA1c closely.
- Cancer Risk: Discontinue use in patients with newly diagnosed or recurrent malignancies. Regular screening is advised for those with a cancer history.
- Injection Technique: Proper training is required to avoid complications like lipoatrophy or infection.
- Pediatric Use: Safety and efficacy not established in children.
- Elderly Patients: Use cautiously due to age-related declines in pituitary function and increased risk of glucose intolerance.
- Research Use: Non-clinical Tesamorelin is for laboratory research only and not for human consumption.
Drug Interactions
- CYP3A4 Substrates: Tesamorelin may reduce the efficacy of drugs metabolized by hepatic/intestinal CYP3A4 enzymes (e.g., prednisone, estrogens, carbamazepine, simvastatin). Monitor for reduced therapeutic effects.
- Glucose-Modifying Drugs: Use caution with medications that affect glucose metabolism (e.g., exenatide, diazoxide, insulin).
- Macimorelin: Avoid concurrent use, as Tesamorelin may interfere with diagnostic tests for GH deficiency.
- Other GHRH Analogs or GH Therapies: Combining with other GH-stimulating agents may lead to excessive IGF-1 levels.
Special Populations
- Pregnancy: Contraindicated due to potential risks of glucose intolerance and fetal harm.
- Breastfeeding: Not recommended; effects on breast milk are unknown.
- Renal/Hepatic Impairment: Limited data; use cautiously and monitor closely.
- Geriatric Use: Increased risk of side effects (e.g., glucose intolerance, edema); start with lower doses if necessary.
Storage and Handling
- Unreconstituted Vials:
- Store Egrifta or Egrifta WR at 2°C to 8°C (36°F to 46°F) in the original Medication Box, away from light and moisture.
- Egrifta WR may be stored at 20°C to 25°C (68°F to 77°F) for up to 3 months.
- Research-grade vials may be frozen at -20°C for long-term storage (up to 12 months).
- Reconstituted Solution:
- Use immediately after mixing.
- If not used, store at 2°C to 8°C and discard within 24 hours. Do not freeze.
- Diluent and Supplies: Store bacteriostatic water, syringes, and needles at 20°C to 25°C (68°F to 77°F).
- Handling Precautions:
- Avoid exposure to excessive heat, direct sunlight, or freezing (unless specified for research-grade storage).
- Check vials for cracks or damage before use; discard if compromised.
- Disposal: Dispose of used needles, syringes, and unused reconstituted solution in a puncture-resistant sharps container. Follow local regulations for biohazardous waste disposal.
Available Formulations
- Egrifta SV (Subcutaneous Vial): 2mg/vial, designed for daily reconstitution and injection.
- Egrifta WR (Weekly Reconstitution): 11.6mg/vial, approved March 25, 2025, allowing weekly reconstitution for daily 2mg doses, improving patient convenience.
- Research-Grade Tesamorelin: Typically 10mg/vial, supplied by peptide manufacturers for laboratory use only (not for human consumption).
Clinical Data and Efficacy
- Pivotal Trials: Two Phase III studies (LIPO-010 and CTR-1011) involving 816 HIV-infected patients with lipodystrophy showed:
- 15–20% reduction in VAT after 26 weeks of 2mg/day dosing.
- Significant reductions in waist circumference (average 2–3 cm) and trunk fat.
- Improvements in triglycerides and HDL cholesterol without significant changes in LDL.
- Sustained effects with continued use up to 52 weeks.
- Safety Profile: Well-tolerated in most patients, with discontinuation rates of ~3% due to adverse events.
- Research Studies: Ongoing trials explore Tesamorelin’s role in NAFLD, cognitive impairment, and obesity, with promising preliminary results.
Warnings and Limitations
- Not for Weight Loss: Tesamorelin is not approved for general weight loss or cosmetic fat reduction.
- Infection Risk: Sharing needles or improper injection technique can transmit infections.
- Research-Grade Use: Non-clinical Tesamorelin is strictly for in vitro or animal studies and not for human consumption.
- Regulatory Compliance: Ensure compliance with local laws when purchasing or using Tesamorelin, especially for research purposes.
- Long-Term Effects: Limited data on use beyond 52 weeks; long-term safety requires further study.
Disclaimer
This product description is for informational purposes only and does not constitute medical advice. Tesamorelin should only be used under the supervision of a qualified healthcare provider for its FDA-approved indication or by licensed professionals for research purposes. Consult a healthcare professional to assess risks, benefits, and appropriate use. Research-grade Tesamorelin is not intended for human consumption and must be handled in accordance with laboratory safety protocols.






Reviews
There are no reviews yet.