Description
Product Overview
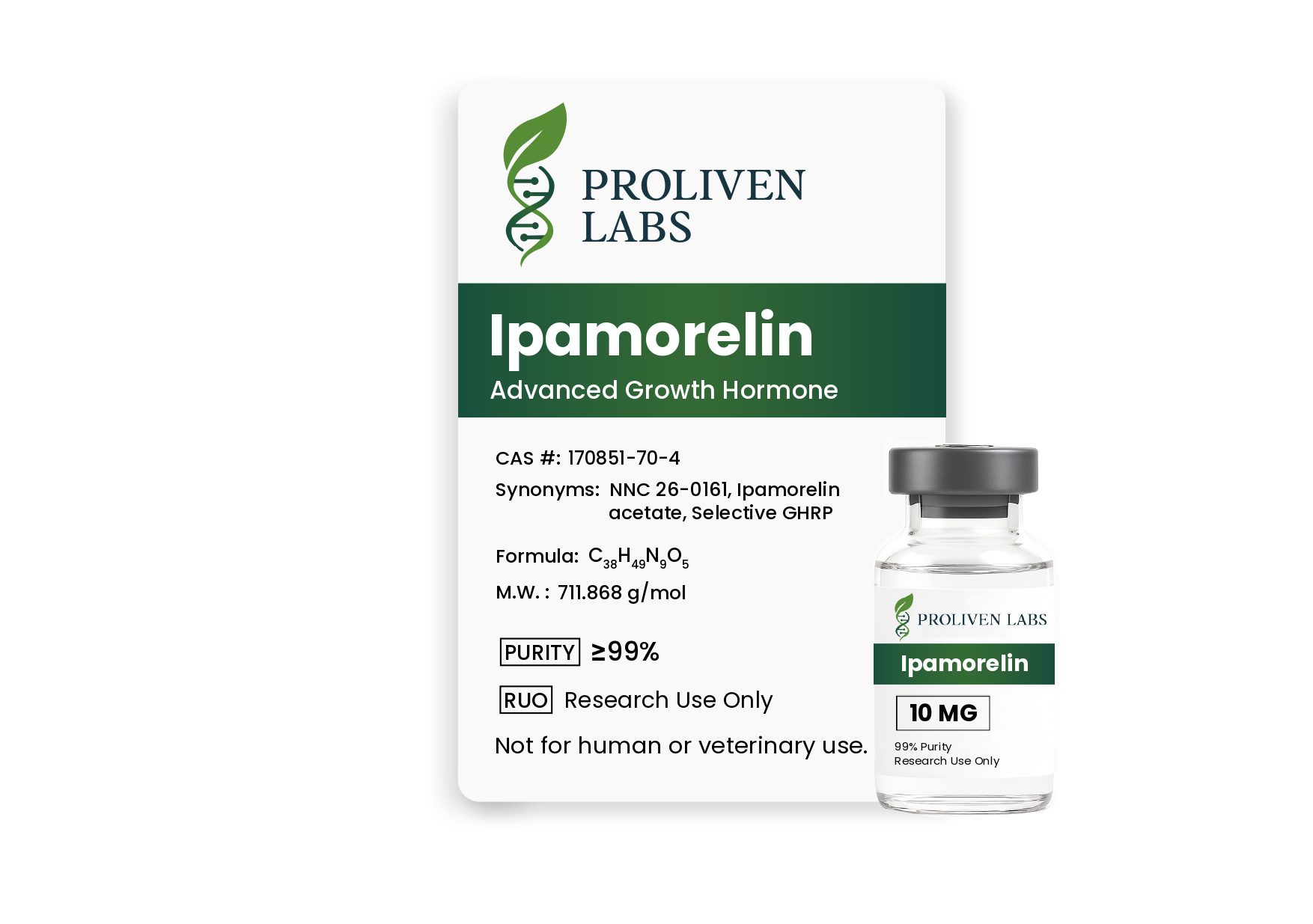
Ipamorelin is a highly purified, synthetic pentapeptide (Aib-His-D-2-Nal-D-Phe-Lys-NH2) developed exclusively for laboratory research. As a selective growth hormone (GH) secretagogue, it targets the ghrelin/growth hormone secretagogue receptor (GHS-R1a) to stimulate pulsatile GH release with exceptional specificity and minimal impact on other hormones, such as ACTH, cortisol, prolactin, follicle-stimulating hormone (FSH), luteinizing hormone (LH), or thyroid-stimulating hormone (TSH). With a molecular formula of C38H49N9O5 and a molecular weight of 711.868 g/mol, Ipamorelin is a premier tool for researchers exploring GH-mediated physiological processes, including muscle growth, metabolic regulation, aging, bone health, and gastrointestinal motility. Supplied as a lyophilized powder with ≥99% purity, this product is strictly intended for in-vitro or controlled preclinical research and is not approved for human consumption, medical, or veterinary use.
Key Features
- Ultra-High Purity: ≥99% purity, verified by reverse-phase High-Performance Liquid Chromatography (HPLC) and mass spectrometry, ensuring consistent, reliable, and reproducible results for rigorous research applications.
- Selective GH Stimulation: Unlike other GH-releasing peptides (e.g., GHRP-2, GHRP-6), Ipamorelin selectively induces GH release without significantly elevating cortisol or ACTH levels, even at doses up to 0.03 mg/kg, offering a cleaner endocrine profile.
- High Potency: Exhibits an EC50 of 1.3 ± 0.4 nmol/L for GH release in primary rat pituitary cells, with a maximal GH production rate of approximately 694 mIU/L/h and dose-dependent increases in insulin-like growth factor-1 (IGF-1) in human and animal models.
- Stable Formulation: Provided as a lyophilized powder (acetate salt) for extended shelf-life and stability, requiring reconstitution with bacteriostatic water (not included) for research use.
- Versatile Research Tool: Ideal for in-vitro and preclinical studies exploring GH dynamics, with applications in muscle recovery, metabolic health, aging, and more.
- Research-Only Product: Exclusively for laboratory use; not intended for human, medical, or veterinary applications.
Chemical and Pharmacological Profile
- Molecular Formula: C38H49N9O5
- Molecular Weight: 711.868 g/mol
- CAS Number: 170851-70-4
- Peptide Sequence: Aib-His-D-2-Nal-D-Phe-Lys-NH2 (pentapeptide structure)
- Mechanism of Action: Acts as a ghrelin mimetic, binding to GHS-R1a receptors to stimulate pulsatile GH release while preserving the body’s natural GH secretion patterns, minimizing endocrine disruption.
- Pharmacological Selectivity: Minimal impact on ACTH, cortisol, prolactin, FSH, LH, or TSH, distinguishing it from less selective GHRPs.
- Potency Metrics:
- EC50: 1.3 ± 0.4 nmol/L for GH release in vitro (rat pituitary cells).
- Emax: 85 ± 5% for GH release.
- SC₅₀: 214 nmol/L for GH release; maximal GH production rate ~694 mIU/L/h.
- Half-Life: Approximately 2 hours in animal models, enabling precise control in time-sensitive experiments.
- Historical Context: Originally developed by Novo Nordisk and evaluated by Helsinn Therapeutics in phase II clinical trials for postoperative ileus (discontinued due to insufficient therapeutic efficacy), Ipamorelin remains a gold standard in GH research.
Research Applications
Ipamorelin’s selective GH-releasing properties make it a versatile peptide for a wide range of scientific investigations. Key research applications include:
- Muscle Growth and Recovery: Supports studies on myoblast differentiation, protein synthesis, and muscle repair pathways. Research indicates Ipamorelin may enhance muscle strength and counteract catabolic effects of glucocorticoids, making it valuable for anabolic studies.
- Metabolic Regulation: Facilitates exploration of GH and IGF-1 effects on fat metabolism, insulin sensitivity, and lean body mass optimization, with potential relevance to obesity and metabolic syndrome research.
- Aging Biology: Investigates GH’s role in age-related declines in muscle mass, bone density, and skin elasticity. Ipamorelin’s ability to mimic natural GH secretion patterns makes it ideal for studying aging processes in preclinical models.
- Gastrointestinal Motility: Ipamorelin’s effects on gastric emptying and postoperative ileus, as demonstrated in rodent models where it accelerated gastric motility.
- Bone Health: Explores its potential to mitigate glucocorticoid-induced bone loss and enhance periosteal bone formation, supporting research into bone mineral content and skeletal health.
- Endocrine Dynamics: Enables precise studies of GH secretion regulation without confounding effects on other hormonal axes, ideal for endocrinology research.
Suggested Protocol
While protocols must be tailored to specific study objectives and institutional guidelines, typical research parameters include:
- Dosage: 200–300 mcg per administration, delivered 2–3 times daily (e.g., 100 mcg per subcutaneous injection) in animal models or in-vitro setups.
- Cycle Duration: 8–12 weeks, followed by a 4–8 week washout period to assess long-term effects and prevent receptor desensitization.
- Administration: Subcutaneous injection after reconstitution with bacteriostatic water. Use sterile techniques (alcohol swabs, sterile syringes, and vials) to ensure safety and accuracy.
- Reconstitution: Dissolve 10 mg vial in 2–5 mL bacteriostatic water to achieve desired concentration (e.g., 2 mL yields 5 mg/mL). Gently swirl to mix; avoid vigorous shaking to preserve peptide integrity.
- Monitoring: Researchers should monitor GH and IGF-1 levels in subjects to correlate with study outcomes, adjusting dosages as needed based on model, age, weight, and research goals.
- Note: Consult peer-reviewed literature and institutional protocols to design experiments. Individual responses may vary based on experimental conditions.
Reconstitution and Storage
- Reconstitution: Add 2–5 mL bacteriostatic water (not included) per 10 mg vial, depending on desired concentration. Gently swirl until fully dissolved; avoid shaking to prevent degradation.
- Storage (Lyophilized): Store at 2–8°C (refrigerated) in a dry, dark environment. Stable for up to 24 months under proper conditions.
- Storage (Reconstituted): Refrigerate at 2–8°C and use within 2–4 weeks to maintain potency. Do not freeze reconstituted solutions.
- Stability Considerations: Protect from light and excessive heat. Use sterile equipment to prevent contamination during reconstitution and handling.
Safety and Handling
- **Int personally for laboratory research; not FDA-approved for human consumption, medical, or veterinary use.
- Side Effect Profile (Research Observations): Limited studies in animal models suggest Ipamorelin is well-tolerated at doses up to 0.03 mg/kg twice daily for 7 days, with no significant endocrine disruptions compared to placebo. Potential side effects include mild flushing, headache, or injection-site irritation, which may subside with dosage adjustment.
- Precautions: Handle with sterile gloves, syringes, and alcohol swabs to avoid contamination. Dispose of all waste (vials, syringes, etc.) in accordance with local biohazard regulations. Researchers should avoid direct exposure and ensure proper ventilation during handling.
- Contraindications: Not applicable for research settings, but researchers should ensure subjects (e.g., animal models) are free from conditions that may confound study outcomes (e.g., pituitary disorders).
- Regulatory Compliance: Researchers are responsible for adhering to local, national, and institutional regulations governing peptide use in research.
Packaging and Specifications
- Quantity: 10 mg per vial
- Form: Lyophilized powder (acetate salt)
- Packaging: Sterile, vacuum-sealed glass vials with rubber stopper and aluminum cap, ensuring product integrity during shipping and storage.
- Purity: ≥99%, verified by third-party HPLC and mass spectrometry.
- Appearance: White to off-white lyophilized powder.
- Solubility: Highly soluble in sterile or bacteriostatic water; insoluble in ethanol, chloroform, or neutral solvents.
- Certificate of Analysis (CoA): Available upon request from reputable suppliers to confirm purity and batch consistency.
- Manufacturing: Produced in cGMP-compliant facilities to ensure quality, consistency, and traceability.
Quality Assurance
- Third-Party Testing: All batches undergo rigorous testing for purity, identity, and potency via HPLC and mass spectrometry.
- Batch Traceability: Each vial is labeled with batch and lot numbers for traceability and quality control.
- Supplier Standards: Reputable vendors adhere to cGMP standards and provide documentation to confirm product authenticity.
Disclaimer
Ipamorelin 10 mg is sold exclusively for in-vitro and preclinical research purposes. It is not intended for human consumption, medical, or veterinary applications. The FDA has not evaluated or approved Ipamorelin for therapeutic use. Researchers are solely responsible for ensuring compliance with all applicable local, national, and institutional regulations. Misuse or unauthorized use is strictly prohibited.


Reviews
There are no reviews yet.