Description
Product Overview
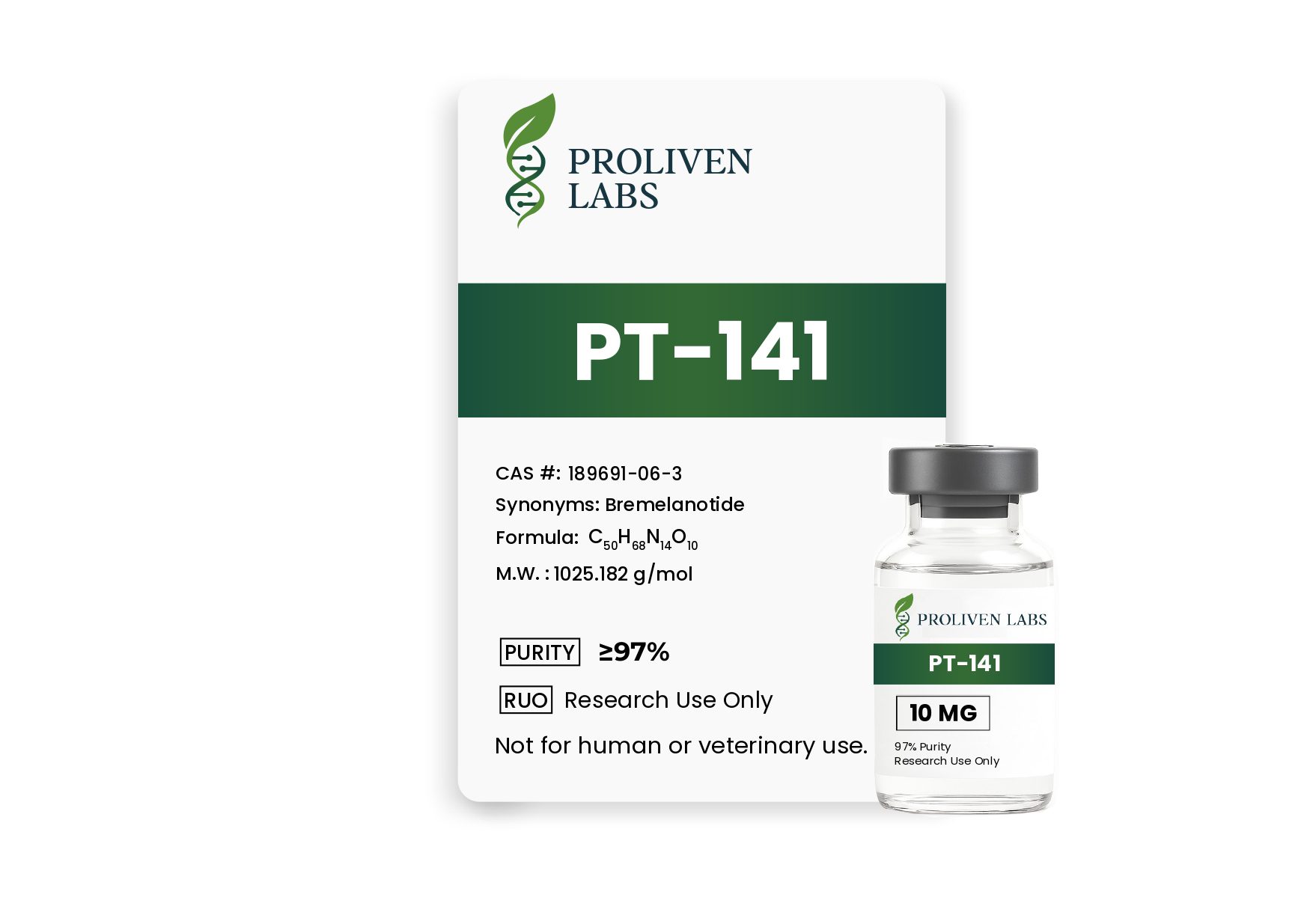
PT-141, scientifically known as Bremelanotide, is a synthetic cyclic heptapeptide and a potent agonist of melanocortin receptors, primarily targeting melanocortin-4 (MC4R) and melanocortin-1 (MC1R) receptors. Originally developed as a derivative of Melanotan II, PT-141 is a heavily modified analog of alpha-melanocyte-stimulating hormone (α-MSH). It has gained prominence for its unique ability to enhance sexual desire and arousal by acting on neurological pathways in the central nervous system, distinguishing it from traditional hormonal or vascular-based therapies. PT-141 is supplied as a 10 mg lyophilized (freeze-dried) powder in a sterile glass vial, intended for reconstitution with bacteriostatic water or sterile saline for research or clinical use under strict medical supervision. This product is designated for in-vitro research or professional medical applications and is not approved for personal use in humans or animals outside of controlled settings. PT-141 received FDA approval in 2019 under the brand name Vyleesi for treating hypoactive sexual desire disorder (HSDD) in premenopausal women, and it shows promise in addressing erectile dysfunction (ED) in men, particularly for those unresponsive to conventional treatments.
Chemical and Physical Properties
- Chemical Name: N-Acetyl-norleucyl-cyclo(aspartyl-histidyl-D-phenylalanyl-arginyl-tryptophyllysine) acetate
- Molecular Formula: C50H68N14O10
- Molecular Weight: 1025.182 g/mol
- CAS Number: 189691-06-3
- PubChem ID: 9941379
- Amino Acid Sequence: Ac-Nle-cyclo[Asp-His-D-Phe-Arg-Trp-Lys]-OH
- Appearance: White to off-white lyophilized powder
- Excipient: Mannitol (stabilizer used during lyophilization to maintain peptide integrity)
- Purity: ≥97% (verified through high-performance liquid chromatography [HPLC] and mass spectrometry by third-party ISO-certified laboratories)
- Solubility: Soluble in sterile water, bacteriostatic water, or saline solution for reconstitution
- pH Stability: Stable at pH 4-7 after reconstitution
- Shelf Life (Unreconstituted): Up to 36 months when stored at ≤5°C (41°F)
- Shelf Life (Reconstituted): Up to 30 days when refrigerated at 2-8°C (36-46°F)
Mechanism of Action
PT-141 functions by selectively binding to and activating melanocortin receptors, particularly MC4R in the brain, which regulates sexual arousal, desire, and emotional responses. Unlike phosphodiesterase-5 (PDE-5) inhibitors (e.g., sildenafil), which target vascular mechanisms to facilitate erections, PT-141 stimulates neurological pathways, promoting libido and sexual arousal in both men and women. This makes it a non-hormonal therapeutic option, ideal for individuals with sexual dysfunction not caused by vascular or hormonal deficiencies. Activation of MC4R enhances dopamine release in the brain, which is associated with sexual motivation and pleasure. Additionally, PT-141’s interaction with MC1R may contribute to secondary effects, such as immune system modulation, potential anti-inflammatory properties, and DNA repair mechanisms, making it a candidate for broader research applications, including obesity management and cancer prevention. Its unique mechanism allows effects to manifest within 30-45 minutes of administration, with peak activity occurring within 2-4 hours.
Intended Applications
PT-141 is primarily researched and used for:
- Hypoactive Sexual Desire Disorder (HSDD): FDA-approved (as Vyleesi) for premenopausal women experiencing distressing low sexual desire not attributable to medical, psychological, or relationship issues.
- Erectile Dysfunction (ED): Demonstrates efficacy in men, particularly those with psychogenic ED or non-responsiveness to PDE-5 inhibitors, by promoting erections with sexual stimulation.
- Research Purposes: Investigated for:
- Immune system stimulation via MC1R activation.
- Obesity treatment, as MC4R deficiencies are linked to weight gain.
- Potential cancer prevention through DNA repair pathways stimulated by melanocortin receptors.
Mood enhancement and stress reduction due to dopamine modulation.
This product is strictly for laboratory research or use under medical supervision and is not intended for personal consumption outside of these contexts.
Dosage and Administration Guidelines
Dosage recommendations are provided for research and clinical reference only and must be determined by a qualified healthcare professional based on individual factors such as body weight, medical history, and intended use.
- Reconstitution:
- Add 1-2 mL of bacteriostatic water or sterile saline to the 10 mg vial to achieve a concentration of 5-10 mg/mL.
- Gently swirl to mix; avoid shaking to prevent peptide degradation.
- Use a sterile syringe for withdrawal and administration.
- Administration Routes:
- Subcutaneous Injection: Preferred method, administered under the skin (e.g., abdomen or thigh) at least 45 minutes before anticipated sexual activity. Use a 1 mL insulin syringe with a 30-gauge needle for minimal discomfort.
- Intranasal: Less common due to variable absorption (historically tested but not standard).
- Oral (Experimental): Some protocols, such as the Maximus LOVER Protocol, combine PT-141 (2000 mcg) with tadalafil (5 mg) in tablet form for synergistic effects, though this is not standard for the 10 mg lyophilized product.
- Dosage Guidelines:
- Initial Dose: 0.5 mg to assess tolerance and response, administered under medical supervision.
- Maintenance Dose: 1-2 mg per administration, adjusted based on efficacy and side effects.
- Body Weight-Based Dosing: 0.25-0.5 mg/kg of body weight, tailored to individual needs.
- Maximum Dose: Do not exceed 2 mg per administration to minimize adverse effects.
- Frequency: Limit to one dose per 24 hours and no more than eight doses per month to reduce risks such as nausea or hyperpigmentation.
- Historical Context: Early clinical trials tested doses up to 10 mg, which caused prolonged erections (priapism) in some subjects, leading to refined dosing at 1.25-2 mg for optimal safety and efficacy.
- Administration Notes:
- Use sterile techniques to prevent contamination.
- Rotate injection sites to avoid tissue irritation.
- Effects may persist for 6-12 hours, with peak activity at 2-4 hours post-administration.
- Sexual stimulation is required for optimal effects in ED treatment.
Storage and Handling
- Unreconstituted Storage:
- Store at ≤5°C (41°F) in a refrigerator, away from heat, moisture, and direct sunlight.
- Keep in a sealed, airtight container to prevent humidity exposure.
- Stable for up to 36 months under proper conditions.
- Reconstituted Storage:
- Store at 2-8°C (36-46°F) in a refrigerator.
- Stable for 3-4 weeks post-reconstitution; discard if solution appears cloudy or discolored.
- Do not freeze reconstituted solution, as this may denature the peptide.
- Shipping Stability: Lyophilized PT-141 remains stable for 3-4 months without refrigeration during transport due to the lyophilization process.
- Disposal:
- Dispose of unused or expired product in a hard, puncture-resistant container to prevent accidental needle sticks.
- Follow local regulations for biohazardous waste disposal.
- Keep out of reach of children and pets.
Potential Benefits
- Sexual Health: Enhances libido and arousal in both men and women, offering a non-hormonal alternative for HSDD and ED.
- Neurological Effects: May improve mood, reduce stress, and enhance emotional well-being through dopamine modulation.
- Research Applications: Shows promise in:
- Immune system stimulation via MC1R activation.
- Obesity management by addressing MC4R-related deficiencies.
- Potential cancer prevention through DNA repair mechanisms.
- Non-Vascular Mechanism: Effective for individuals unresponsive to PDE-5 inhibitors, as it targets neurological pathways rather than blood flow.
- Rapid Onset: Effects begin within 30-45 minutes, with peak activity at 2-4 hours, providing a convenient therapeutic window.
Potential Side Effects and Precautions
- Common Side Effects:
- Nausea (most frequent, reported in ~40% of users at higher doses).
- Flushing or warmth (temporary).
- Headache or dizziness.
- Injection site reactions (redness, swelling, or pain).
- Hyperpigmentation (rare, associated with prolonged or high-dose use due to MC1R activation).
- Serious Side Effects (Rare):
- Prolonged erections (priapism) in men, particularly at doses >2 mg. Seek immediate medical attention if an erection lasts >4 hours.
- Increased blood pressure (monitor in patients with cardiovascular conditions).
- Allergic reactions (e.g., rash, swelling, difficulty breathing).
- Contraindications:
- Not for use in postmenopausal women, men (for HSDD), or children, as safety and efficacy are not established.
- Avoid in patients with uncontrolled hypertension or cardiovascular disease, as PT-141 may increase blood pressure.
- Use caution with medications affecting blood pressure or cardiovascular function.
- Precautions:
- Disclose all medical conditions (e.g., heart disease, kidney/liver issues) and medications to a healthcare provider before use.
- Avoid alcohol consumption near administration, as it may exacerbate side effects like nausea or dizziness.
- Monitor for signs of hyperpigmentation with prolonged use.
- Not suitable for pregnant or breastfeeding women due to insufficient safety data.
- Allergies: Inform healthcare providers of allergies to peptides, mannitol, or related compounds.
Packaging and Availability
- Quantity: 10 mg per sterile glass vial (lyophilized powder).
- Packaging: Sealed in a 2 mL or 5 mL glass vial with a rubber stopper and aluminum cap to ensure sterility.
- Formats: Available as single vials or multi-vial packs for research purposes.
- Quality Assurance:
- Manufactured in ISO-certified facilities with Good Manufacturing Practices (GMP) compliance.
- Purity ≥97%, verified by HPLC and mass spectrometry.
- Each batch includes a Certificate of Analysis (CoA) from third-party testing.
Legal and Regulatory Information
- Intended Use: Sold exclusively for in-vitro research or use under medical supervision (e.g., prescribed as Vyleesi for HSDD). Bodily introduction into humans or animals outside of these contexts is prohibited by law.
- FDA Status: Approved as Vyleesi for HSDD in premenopausal women (2019). Not approved for other indications or populations without further research.
- Research Use Disclaimer: Suppliers are not responsible for misuse, misadministration, or adverse effects resulting from improper handling or use outside of research or medical protocols.
Warnings and Disclaimers
- This product is not intended to diagnose, treat, cure, or prevent any disease without professional medical guidance.
- Always consult a licensed healthcare provider before use, especially for individuals with pre-existing conditions or those taking medications.
- Do not disregard or delay seeking medical advice based on this information.
- Misuse may result in serious health risks, including cardiovascular complications or priapism.
- Keep out of reach of children and unauthorized individuals.
The manufacturer and supplier assume no liability for improper storage, handling, or administration.


Reviews
There are no reviews yet.